|
|
|
Synthesis
|
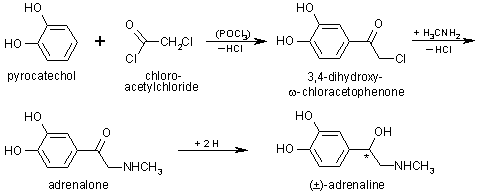
 The first total chemical synthesis of adrenaline was performed in 1904
by F. Stolz et al. pyrochatechol and chloro-acetylchloride react in the presence
of phosphorchloridoxide to 3,4-dihydroxy-
The first total chemical synthesis of adrenaline was performed in 1904
by F. Stolz et al. pyrochatechol and chloro-acetylchloride react in the presence
of phosphorchloridoxide to 3,4-dihydroxy-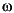 -chloracetophenone (Fries-rearrangement). After the conversion with
methylamine, adrenalone will be reduced with sodiumamalgam to adrenaline.
-chloracetophenone (Fries-rearrangement). After the conversion with
methylamine, adrenalone will be reduced with sodiumamalgam to adrenaline.
Severall other syntheses also use adrenalone as starting material.

|
|
Biosynthesis
|
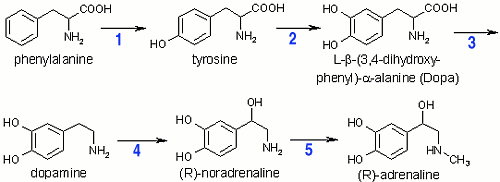
 1: Phenylalanine-hydroxylase,
2:
Tyrosine-hydroxylase,
3:
Aromatic amino-acid decarboxylase,
4:
Dopamine-
1: Phenylalanine-hydroxylase,
2:
Tyrosine-hydroxylase,
3:
Aromatic amino-acid decarboxylase,
4:
Dopamine-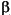 -hydroxylase,
5: Phenylethanolamine- N-methyl- transferase -hydroxylase,
5: Phenylethanolamine- N-methyl- transferase
Five enzymes are involved in the pathway of the biosynthesis of adrenaline.
The first enzyme is the iron containing phenylalanine-hydroxylase (also called
phenylalanine-4-monooxygenase). The second enzyme, tyrosine-hydroxylase, contains iron,
too, and catalyses the conversion of tyrosine to
L-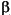 -(3,4-dihydroxyphenyl)- -(3,4-dihydroxyphenyl)-
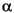 -alanine (dopa). After
decarboxylation of dopa to dopamine (aromatic amino-acid decarboxylase) the
copper-containing enzyme dopamine- -alanine (dopa). After
decarboxylation of dopa to dopamine (aromatic amino-acid decarboxylase) the
copper-containing enzyme dopamine-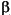 -hydroxylase converts dopamine to noradrenaline. The final enzyme
noradrenaline-N-methyltransferase then methylates noradrenaline to adrenaline.
The biosynthesis occurs in the adrenal medulla (see biologic
function).
-hydroxylase converts dopamine to noradrenaline. The final enzyme
noradrenaline-N-methyltransferase then methylates noradrenaline to adrenaline.
The biosynthesis occurs in the adrenal medulla (see biologic
function).

|
Last change: 1999-04-12
Frank.Oellien@chemie.uni-erlangen.de
|